- What is Specific Gravity?
- Definition of “Specific Gravity” in a Battery
- What is meant by battery acid?
- Why is it important to measure the specific gravity and density of battery acid?
- Methods for measuring battery acid
- Measuring Specific Gravity with a hydrometer
- Measuring Specific Gravity with a digital density meter
- Temperature correction for the specific gravity of acid
- Open-Circuit Voltage Test
What is Specific Gravity?
The term “specific gravity” refers to the ratio that is obtained by comparing the weight of any liquid to the weight of an equivalent volume of water. This comparison can be done for any liquid.
Definition of “Specific Gravity” in a Battery
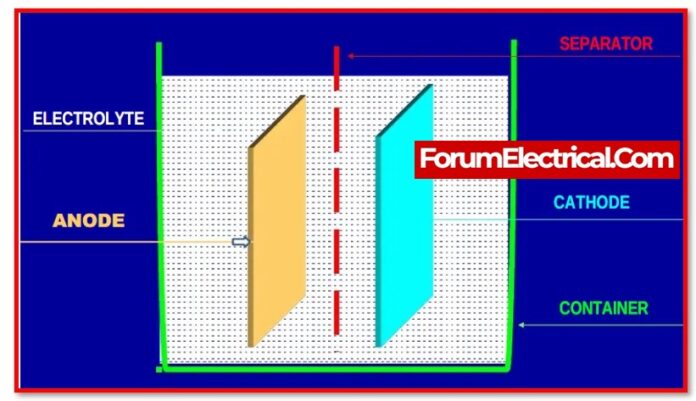
The weight of the electrolyte in comparison to the weight of the same volume of pure water It is utilised for the purpose of determining the strength of the sulfuric acid present in the electrolyte or the percentage of its presence.
Pure water has a specific gravity of 1.000.
Lead-acid batteries employ a sulfuric acid-containing electrolyte. Because pure sulfuric acid weighs 1.835 times as much as pure water per unit volume, it has a specific gravity of 1.835.
Because the electrolyte of a lead-acid battery is a mixture of water and sulfuric acid, its specific gravity will fall between 1.000 and 1.835. The electrolyte for a battery is typically prepared so that the specific gravity is less than 1.350.
What is meant by battery acid?
The electrolyte that is present in batteries is referred to as “battery acid”. The acid is sulfuric acid, which is used for lead acid batteries (H2SO4). Sulfuric acid is a powerful acid that is odourless, colourless, and acidic.
Why is it important to measure the specific gravity and density of battery acid?
The level of charge can be estimated by looking at the specific gravity of the electrolyte that is contained within the battery. The density of the sulfuric acid electrolyte, also known as its specific gravity, drops as a result of chemical processes that take place during discharge.
When the density of the battery acid is measured, the concentration of H2SO4 can be determined, in addition to the charging status of the battery.
The operator understands whether the battery only needs little maintenance or whether it needs to be replaced altogether based on the results.
A regular examination of the battery’s density is required in order to locate and maintain the cells that are the least efficient.
Methods for measuring battery acid
The specific gravity of the electrolyte can be determined using
- A hydrometer (also known as an “aerometer”)
- A digital density meter (sometimes known as a “digital hydrometer”).
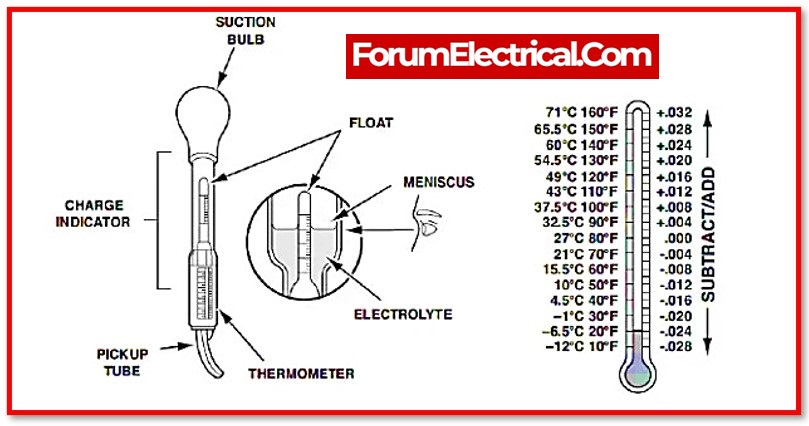
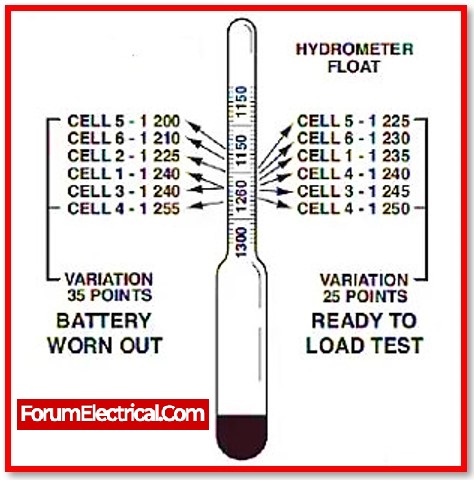
Measuring Specific Gravity with a hydrometer
Hydrometers (aerometer) are used to determine the specific gravity of liquids. The specific gravity of a liquid is the ratio of its density to that of water. In the function of battery testing, the hydrometer measures the specific gravity of the electrolyte in the battery. The higher the specific gravity, the higher the acid content in the electrolyte.
The user can determine the battery’s state of charge based on its specific gravity.
IEEE recommends storing hydrometer readings and data over time as part of any Battery Maintenance Program.
A lead acid battery hydrometer is a specialised kind of hydrometer that resembles a syringe with a bulb attached to the end of it. A float that can be calibrated for measuring specific gravity can be found inside the bulb (SG).
To use the hydrometer, first remove some of the battery acid, which is represented by the chemical formula H2SO4, and then transfer that acid into the bulb. After that, read the value that is displayed on the float that is floating in the sample.
Measuring Specific Gravity with a digital density meter
As long as the measuring cell can withstand aggressive acids, a digital density meter (also known as a digital hydrometer) can be used to determine the specific gravity of the sulfuric acid electrolyte. The result is often converted to the correct temperature and presented on the digital display in the relevant unit, such as SG (Specific Gravity) 80/80.
Density meters (also known as digital hydrometers) provide the following advantages over hydrometers:
- Cleaning is simpler as well as faster and provides the result on a digital display that has already been converted to temperature.
- Because the samples are identical, cleaning the cell between measurements using a digital density meter is not required; just rinsing prior to the next measurement is conducted.
- A density meter simply requires the handling of a small volume of 2 mL to measure density.
- It is feasible to achieve acceptable density measurement findings by following a few simple rules.
Using the specific gravity in the following table, one may determine the depth of discharge (DOD) of the cell of the battery that the electrolyte was taken from.
| DOD (Depth of Discharge) | 2V Battery | 12V Battery | 24V Battery | 48V Battery | Specific Gravity of Battery |
|---|---|---|---|---|---|
| 0% | 2.10 | 12.70 | 25.40 | 50.80 | 1.26 |
| 10% | 2.09 | 12.58 | 25.16 | 50.32 | 1.25 |
| 20% | 2.08 | 12.46 | 24.92 | 49.84 | 1.23 |
| 30% | 2.06 | 12.36 | 24.72 | 49.44 | 1.22 |
| 40% | 2.05 | 12.28 | 24.56 | 49.12 | 1.20 |
| 50% | 2.03 | 12.20 | 24.40 | 48.80 | 1.19 |
| 60% | 2.02 | 12.12 | 24.24 | 48.48 | 1.17 |
| Discharged | 1.75 | 11.90 | 23.80 | 47.60 | 1.12 |
Temperature correction for the specific gravity of acid
The SG of acid changes depending on the temperature. When the temperature is extremely cold or extremely hot, this can cause the readings to be inaccurate. Use the following calculations to correct for temperature, or remove points at a rate of 0.003 per 10 degrees Fahrenheit if the temperature is below 70℉, and add points if the temperature is above 70 ℉.
- The correction factor = (0.331 x Cell Temp ℉ – 23)/1000, which is equal to 0.003 points for every 10℉.
- The correction factor = (0.595 x Cell Temperature°C – 12.5)/1000.
- This is accurate from -17.8 – 54.4° C to 0-130 ℉.
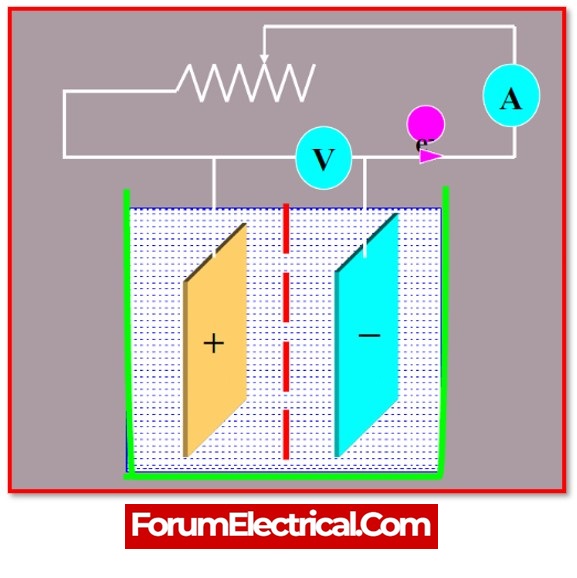
Open-Circuit Voltage Test
Batteries need to be left idle (not charged, not discharged) for at least six hours, and preferably for twenty-four hours, before correct voltage readings can be obtained.
- Disconnect all of the loads that are connected to the batteries.
- Use a DC voltmeter to get an accurate reading of the voltage.
- Use the table below to get an estimate of the current charge level.
- If the battery shows that it is between 0% and 70% charged, charge it.
- If the battery readings are lower than those shown in the table, one of the following conditions may be present:
- The battery was allowed to remain in a discharged state for an excessive amount of time.
- One of the cells in the battery is defective.
Batteries in this state either need to be taken to a specialist to undergo further testing or they should be removed from service altogether.
The following table illustrates the relationship between the state of charge and specific gravity and open circuit voltage.
| Percentage (%) Of Charge | 12V Battery | 24V Battery | 48V Battery |
|---|---|---|---|
| 100% | 12.73 | 25.46 | 50.93 |
| 90% | 12.62 | 25.24 | 50.47 |
| 80% | 12.50 | 25.00 | 49.99 |
| 70% | 12.37 | 24.74 | 49.49 |
| 60% | 12.24 | 24.48 | 48.96 |
| 50% | 12.10 | 24.20 | 48.41 |
| 40% | 11.96 | 23.92 | 47.83 |
| 30% | 11.81 | 23.63 | 47.26 |
| 20% | 11.66 | 23.32 | 46.63 |
| 10% | 11.51 | 23.02 | 46.03 |