Table of Contents
- What are the essential steps for battery system start-up and commissioning?
- Battery System Start-Up and Commissioning Procedure
- Pre-Startup and Commissioning Checks/Verification
- What are the key pre-startup and commissioning checks for a battery system?
- Initial Electrolyte Filling
- Initial Charging Procedures
- Cell Voltage and Specific Gravity Measurements
- What is the relationship between cell voltage and state of charge?
- Voltage Ratings for Different Battery States
- How does specific gravity change with state of charge?
Starting up and commissioning a battery system is a crucial process to ensure the reliable and efficient operation of the batteries. In this section, we will discuss the essential steps and considerations for battery system start-up and commissioning:
What are the essential steps for battery system start-up and commissioning?
1. Pre-Startup and Commissioning Checks/Verification
2. Initial Electrolyte Filling
3. Initial Charging Procedures
4. Cell Voltage and Specific Gravity Measurements
Battery System Start-Up and Commissioning Procedure
Pre-Startup and Commissioning Checks/Verification
Pre-startup and commissioning checks/verification is essential steps in ensuring the proper installation and reliable operation of a battery system.
What are the key pre-startup and commissioning checks for a battery system?
Battery Frame Inspection
- Verify that the battery frame is assembled in accordance with the manufacturer’s recommendations.
- Ensure that the battery frame is securely anchored to the floor, preventing any movement or instability.
Battery Rack Acid-Proof Paint
- Check the battery rack for any nicks or chips in the acid-proof paint.
- If any chips or nicks are found, use touch-up paint provided by the battery rack manufacturer to cover them.
- Failure to touch up these imperfections could lead to structural damage and even the collapse of the battery rack if corrosive electrolyte is spilled on it.
Cell Support Channels
- Verify that the battery cells rest on plastic or wood support channels.
- These support channels serve to electrically insulate the cell from the steel frame, preventing electrical issues and corrosion.
Cell Inspection
- Inspect each battery cell for signs of electrolyte leakage, which can result from a cracked jar or broken seal.
- Replace any damaged cells to prevent further leakage or contamination.
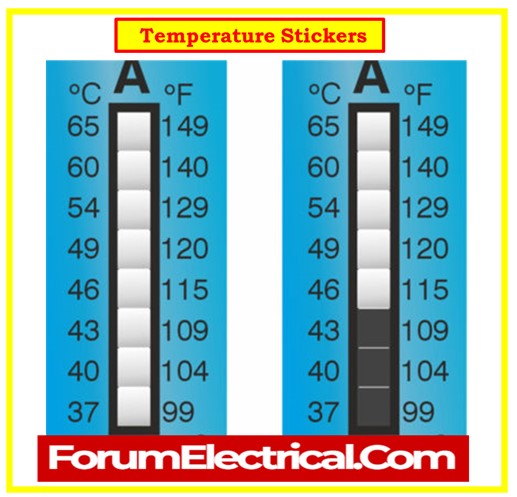
Temperature Stickers
- Check the color change temperature sticker applied to the exterior of each cell.
- If the color changes, indicating that the cell temperature has exceeded 45°C, replace the affected cells immediately to avoid potential issues.
Flame Arrestor and Vent Caps
- Verify that the flame arrestor or explosion-resistant vent caps are intact and properly installed.
- These components collect electrolyte spray and return it to the cell, preventing potential hazards.
Connection Hardware
- Inspect each connection for the correct complement of fastening hardware.
- Correct any connections that do not comply with the manufacturer’s installation instructions.
- Use a insulated torque wrench to apply the correct torque to each battery connection, following the manufacturer’s recommendations for torque values.
Anti-Oxidant Coating
- Ensure that all terminal connector bolts, nuts, and washers have been coated with an anti-oxidant.
- This coating helps prevent corrosion and ensures good electrical connections.
Resistance Measurements
- Utilize the manufacturer’s test instructions to collect field data that establishes the initial connection resistance values of each battery post connection.
- These baseline resistance values are crucial for future maintenance procedures and are recorded in the Individual Cell Terminal Resistance Record.
Voltage Polarity Check
- After completing the inter cell connections, use a voltmeter to check the polarity of the series connections.
- The total voltage of the series string should read approximately twice the number of cells (e.g., 2 x 60 cells = 120 volts).
- If the voltage is too low, it suggests improper cell connections.
- Inspect the battery string immediately and correct any faulty connections to ensure proper operation.
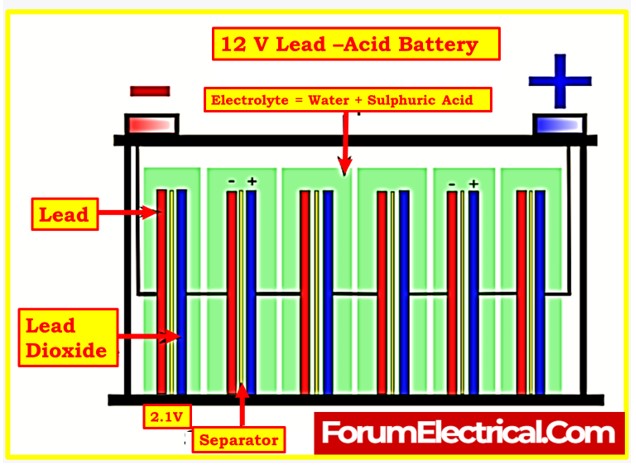
Initial Electrolyte Filling
- Properly filling batteries with the right electrolyte, whether they come with electrolyte or are dry-charged, is essential to ensure safe and efficient operation.
- Following manufacturer guidelines, measuring specific gravity, and taking safety precautions are crucial for the longevity and performance of battery systems.
- Various aspects of the initial electrolyte filling process for batteries, both when they are received with electrolyte and when they are shipped dry-charged.
Shipped with Electrolyte
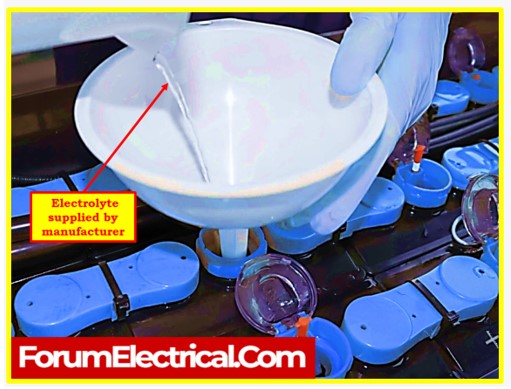
Verification of Electrolyte Level
- When batteries are received from the manufacturer with electrolyte already filled, the first step is to verify the electrolyte level.
- Each cell should be checked to ensure that the electrolyte level is within the manufacturer’s specified range.
- Correct electrolyte levels are crucial for the batteries to function optimally. If the levels are too low, the cells may not perform as expected, and if they are too high, there’s a risk of electrolyte overflow when charging.
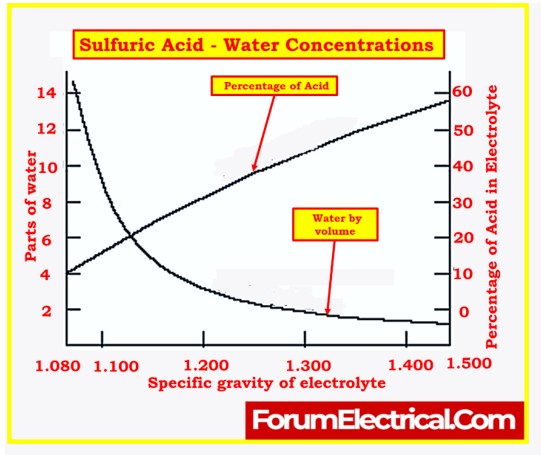
Specific Gravity Measurement
- Another important parameter to check is the specific gravity of the electrolyte in each cell.
- The specific gravity of the electrolyte provides information about its density compared to pure water.
- It is an indicator of the state of charge and the concentration of acid in the electrolyte. The specific gravity should be measured using a hydrometer.
- Consistency in specific gravity among all cells is essential to ensure that the batteries are uniform in their state of charge.
Shipped Dry-Charged
Verification of Seals and Vent Caps
- When batteries are shipped dry-charged, the Electrical Engineer plays a key role in overseeing the initial electrolyte filling process.
- Before adding electrolyte, it’s vital to verify that the cells are sealed and that the moisture vent caps are in place.
- This step ensures that the cells are in good condition and not contaminated.
- A missing or broken vent cap could indicate that the cell is compromised and could lead to problems in the future.
Filling Cells to the Correct Level
- Each cell should be filled to the correct level before proceeding with the freshening charge.
- The level to which each cell should be filled depends on the manufacturer’s recommendations.
- Overfilling or under filling cells can lead to improper battery performance.
Choosing the Correct Electrolyte
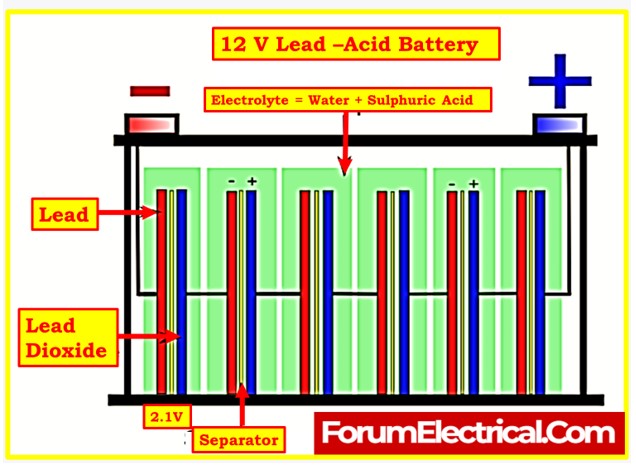
- The choice of the correct electrolyte is crucial. Lead-acid cells typically use an electrolyte composed of sulfuric acid and demineralized water, while nickel-cadmium cells use an electrolyte made from potassium hydroxide and demineralized water.
- The concentration levels of these components are determined by the cell manufacturer and should be strictly followed to ensure optimal performance and longevity of the batteries.
Quality of Demineralized Water
- Demineralized water is essential because it prevents the formation of foreign mineral deposits on the cell plates that could contaminate the cell and reduce its lifespan.
- Demineralized water used for preparing the electrolyte should meet specific purity standards. It should be free from suspended matter, colorless, and have low levels of impurities such as total solids, chloride, iron, copper, manganese, lead, calcium, magnesium, and zinc.
Mixing Electrolyte
- To create the correct electrolyte mix, the concentration of acid in the electrolyte is determined through measurement of specific gravity.
- Specific gravity is a measure of the density of the electrolyte compared to demineralized water.
- The specific gravity of the diluted sulfuric acid will be a result of mixing various amounts of demineralized water (specific gravity 1.000) and sulfuric acid (specific gravity 1.835).
- Mixing must be done with care and attention to safety. Mixing should take place in a properly ventilated area.
- Safety precautions must be followed, including wearing appropriate personal protective equipment, having eyewash stations available, and providing extra water supplies in case of electrolyte spill.
- This is particularly important because mixing the two substances produces heat, and if done incorrectly, it can lead to a violent reaction.
- The exact specific gravity required for a given cell will be specified by the manufacturer, usually falling in the range of 1.215 to 1.250 for lead-acid batteries.
Electrolyte Filling
- After selecting the proper electrolyte and mixing it accurately, the electrolyte can be added to the cells.
- Filling each cell to the lower fill line initially is essential because the level of the electrolyte can vary with temperature.
- The cells should be allowed to sit for a specified time, as recommended by the manufacturer, to allow the plates to absorb electrolyte and for the electrolyte temperature to stabilize.
Adjusting Electrolyte Level
- After the soak time, the electrolyte temperature should be checked. Depending on the design and volume of the cells, the electrolyte may expand or contract with changes in temperature.
- The manufacturer’s specifications should be consulted to ensure the electrolyte level is within the correct range.
- Adjustment of the electrolyte level is required to ensure it falls within the operational range indicated by upper and lower fill lines, accounting for the expansion and contraction of the electrolyte with temperature changes.
Initial Charging Procedures
What are the initial charging procedures for batteries?
- The initial charging procedures for batteries are a crucial step in preparing them for service.
- These procedures vary depending on the type of battery cells, such as lead-antimony and lead-calcium batteries. Let’s delve into the details of these initial charging processes:
Wet Cells and Periodic Monitoring
- Batteries shipped wet or filled in the field may have lost some charge during shipping and installation.
- To bring these batteries to their fully charged state, an initial charging procedure, often referred to as a “freshening charge,” is necessary.
- Wet cells are typically shipped in a fully charged state, but the freshening charge is applied to ensure that the cells are indeed at their optimal charge level.
- It is essential to periodically monitor the state-of-charge of wet cells by measuring the specific gravity.
- If there is a 25 point (0.025) decrease in specific gravity, a freshening charge should be applied to maintain the battery’s performance.
- Cells not immediately placed in service should also undergo periodic freshening charges, as recommended by the battery manufacturer to prevent self-discharge and performance degradation during storage.
Shelf Storage Durations
- Battery shelf storage durations are determined by the type of cell and the electrolyte status (wet or dry charged).
- Wet cells need periodic charges during storage. Storage periods may vary by cell type. For instance, stored lead-antimony cells require a periodic charge every three months, while lead-calcium cells require a freshening charge every six months.
- It’s essential to adhere to these storage duration guidelines to ensure that batteries are in good condition when needed for service.
Lead-Antimony Batteries
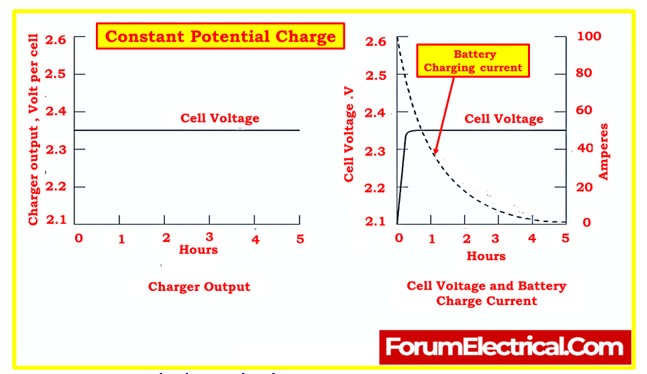
- Lead-antimony batteries require an initial charge within three months of the date of shipment from the manufacturer. This initial charge is called a freshening charge and is usually applied using the constant potential charge method.
- The constant potential charge involves applying a constant voltage to the battery terminals for a specified duration, as recommended by the manufacturer.
- Monitoring of the charge involves observing the cell voltage and charge current. As the charge progresses, the voltage rises to match the charger output voltage, and the charging current decreases.
- The freshening charge should continue at the manufacturer’s specified voltage, usually the equalize voltage, for the specified duration to ensure the cells are fully charged.
Lead-Antimony Battery Charging
- The initial charge voltage per cell (VPC) is determined by dividing the total system voltage by the number of cells in series.
- During the initial charge, a pilot cell should be monitored to prevent the electrolyte temperature from exceeding 49°C. If it does, the charge must be stopped, and the batteries allowed to cool before restarting.
Lead-Calcium Batteries
- Lead-calcium cells have a lower self-discharge rate and can maintain their charged state for a longer time, so the initial charge can be delayed up to six months after shipment.
- The initial charge for lead-calcium batteries should be performed using an initial charge voltage per cell (VPC) corresponding to the nominal specific gravity of the battery.
- The duration of the initial charge is not fixed and continues until the lowest individual cell voltage ceases to rise. Afterward, the charge is maintained for an additional 24 hours.
Monitoring During the Initial Charge
- During the initial charge of lead-calcium batteries, individual cell voltage values must be monitored to identify when the lowest individual cell voltage stops rising.
- The temperature of pilot cells should also be monitored to ensure that the electrolyte temperature remains within acceptable limits, similar to lead-antimony batteries.
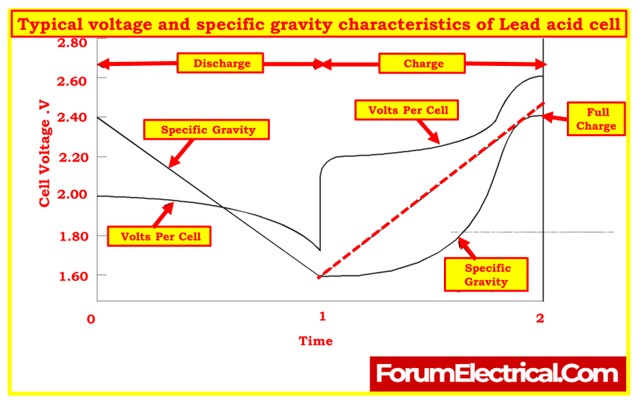
Cell Voltage and Specific Gravity Measurements
- Measuring cell voltage and specific gravity are essential steps in assessing the condition and performance of battery cells.
- These measurements provide valuable information about the electrochemical state of the cells and help determine their capacity. Let’s delve into these measurements in more detail:
What is the relationship between cell voltage and state of charge?
Cell Voltage
- Cell voltage refers to the electrical potential difference between the positive and negative terminals of a battery cell.
- Cell voltage is measured during an open-circuit condition, meaning there is no load connected to the cell. This measurement provides a true indication of the cell’s standard potential or theoretical voltage.
- Theoretical voltage or standard potential is calculated based on the electrode potentials (oxidation potential of the anode and reduction potential of the cathode), the composition of the electrolyte, and the electrolyte temperature.
- Under standard conditions (cell temperature at 25°C and specific gravity within the normal operating range), the open-circuit voltage closely approximates the theoretical voltage.
- The open-circuit voltage of a cell varies based on the state of the cell’s static charge. There’s a relationship between the open-circuit voltage and the concentration of the electrolyte.
- Ideally, a new battery cell on open-circuit should approach the theoretical standard potential. However, over time, as the electrolyte can become contaminated and battery plates corrode, the cell’s voltage will decrease.
- Some contamination issues can be corrected through equalizing charges followed by deep discharge cycles, which can restore the cell’s voltage. If these cycles have no effect and the voltage continues to decrease, the cell may need replacement.
- Individual cell voltage readings can be obtained using a voltmeter connected across the positive and negative terminals. It’s essential to ensure a correct measurement because it directly impacts the assessment of a cell’s condition.
Voltage Ratings for Different Battery States
- Battery cells have various voltage ratings, which reflect their electrical condition and their voltage from fully charged to discharged. These ratings differ based on the operating environment, state of charge, and cell composition.
- For typical lead-acid and nickel-cadmium batteries, the individual cell voltage ratings include:
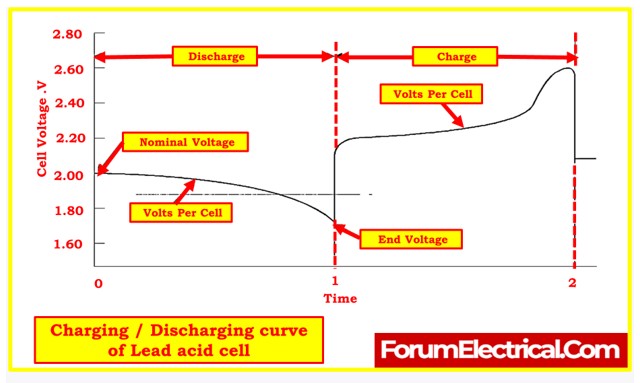
- Open-Circuit Voltage: The potential difference between the terminals when the battery is at no-load.
- Nominal Voltage: The characteristic operating voltage or rated voltage of the cell.
- Working Voltage: Represents the actual operating voltage under load.
- End Voltage: A point on the discharge curve beyond which no usable energy can be drawn.
Monitoring Cell Voltage
- By closely monitoring individual cell voltage during operation and charging, an Electrical Engineer can assess the general condition of any cell in the battery.
- The cell voltage curve during a battery discharge and charge cycle can help identify how cells are responding. Any deviations from expected behavior, such as a cell failing to respond in a similar manner to others, may indicate a defective cell that requires maintenance or replacement.
How does specific gravity change with state of charge?
Specific Gravity
- Specific gravity is a measure of the density of the electrolyte compared to the density of pure water. It provides insights into the state of charge and the chemical reactions occurring within the cell.
- During battery discharge, the density of the electrolyte decreases due to the disassociation of sulfuric acid molecules, resulting in lower specific gravity.
- During a battery charge, sulfuric acid is recombined, increasing the specific gravity of the electrolyte.
- The specific gravity of the electrolyte is closely related to the state of charge, and it changes as a result of the electrochemical reactions taking place in the cell.
- Monitoring specific gravity alongside cell voltage provides a comprehensive understanding of the cell’s state of charge and condition, making it one of the most reliable indicators of a cell’s state of charge.