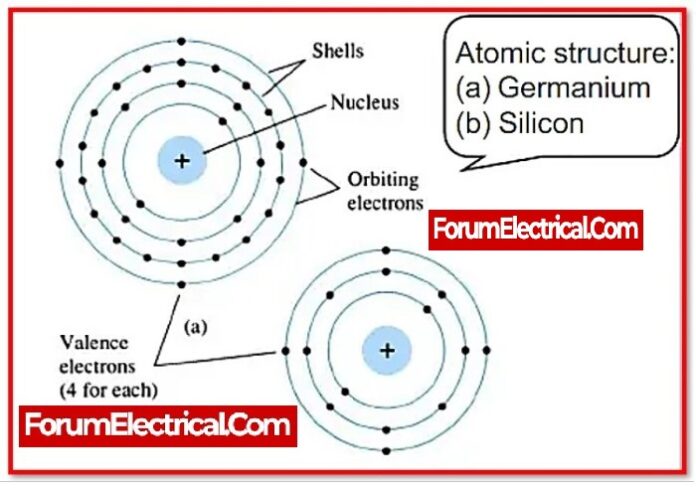
What is Semiconductor?
Materials with a conductivity that ranges between that of conductors and that of insulators or non-conductors are known as semiconductors. Electrical conduction is a process that takes place in semiconductors and involves both the conduction band electron and the valence band hole. Semiconductors can take the form of pure elements like germanium or silicon, or they can take the form of compounds like gallium arsenide.
Types of semiconductors
Semiconductors are classified into two types:
1).Intrinsic semiconductors and
2).Extrinsic semiconductors.
1). Intrinsic semiconductors
Intrinsically pure and impurity-free semiconductors are known to be as intrinsic semiconductors.
Intrinsic semiconductors, also known as pure or undoped semiconductors, are flawless crystals free of the flaws and impurities of other elements.
All semiconductor materials, including all those doped with other components, have intrinsic properties, with the doping components adding other desired qualities.
Intrinsic means inherent & natural, and intrinsic semiconductors exhibit the bulk properties of semiconductor substances rather than impurities and dopants.
Because silicon & germanium are elemental semiconductors, they are most commonly used as intrinsic semiconductors.
They were among the first semiconductors to be widely researched and used.
Semiconductors are distinguished by their electrical structure.
One of the mechanisms that distinguishes semiconductors as a distinct type of material is the electrical structure, that determines the essential properties of semiconductors.
2). Extrinsic semiconductors
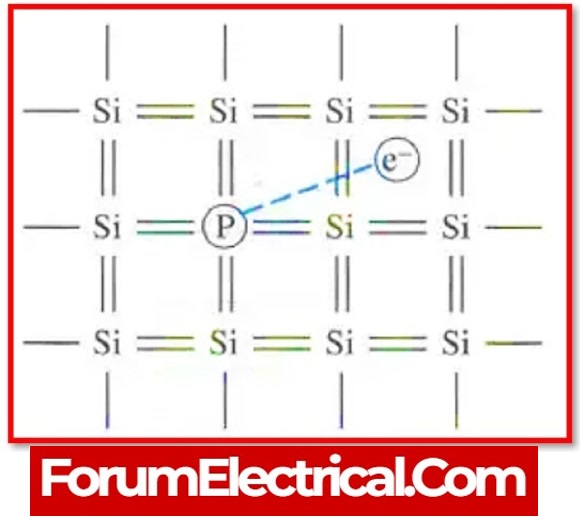
The conductivity of semiconductors can be improved by adding a small number of appropriate atoms known as impurities.
Doping is the process of introducing impurities into a pure semiconductor. In a doped semiconductor, one atom out of every 107 is replaced by the dopant atom.
Extrinsic Semiconductors are classified into two types:
a). N-Type Semiconductor.and
b). P-Type Semiconductor
a). N-Type Semiconductor
When a small (tiny) amount of impurity is introduced into a pure semiconductor, it produces a large number of free electrons.
The resulting extensive semiconductor is known as an n-type semiconductor.
Electrons become the majority carriers in an extrinsic semiconductor with pentavalent impurity, while holes become the minority carriers.
As a result, these are referred to as n-type semiconductors.
b). P-Type Semiconductor
A p-type semiconductor is an extrinsic semiconductor that has been doped with the electron acceptor atoms.
This type of semiconductor is identified from the fact that the majority of charge carriers in the crystal are (+) positive holes.
In p-type semiconductor, the number of holes – greater (>) than the number of electrons.
Because of this, the current can travel along the material from hole to hole, but it can only flow in one direction.
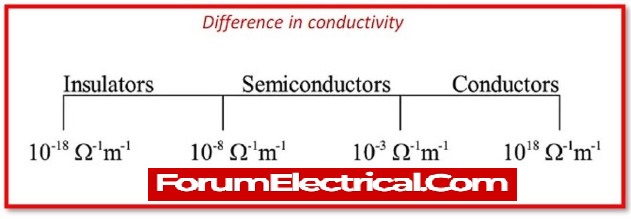
Conductivity of Semiconductor
A material’s conductivity is determined by the concentration of free electrons in it. Insulators have a low concentration of free electrons, whereas good conductors have a high concentration of free electrons.
The conductance of these conductors is very high and have a a low resistance value.
1). Significance of electrons in semiconductor
The concentration of free electrons in semiconductors is between the densities of free electrons in conductors and insulators.
As a result, the semiconductor’s conductivity is moderate, not extremely high or extremely low.
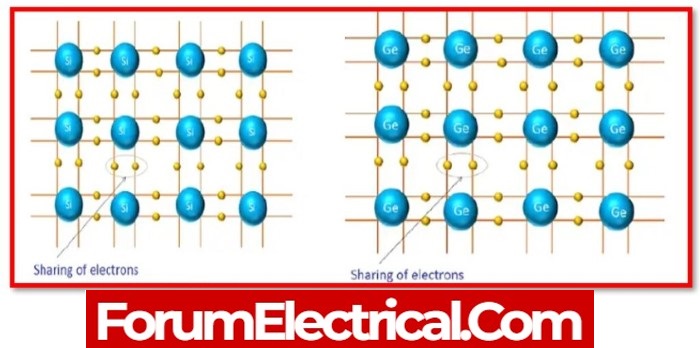
The valance electrons in a semiconductor are not free like those in metal; instead, they become enveloped in the bond between two adjacent atoms.
- Germanium &
- Silicon
are two widely used semiconductor materials. Both semiconductors have a regular repetition of the three-dimensional unit in their crystal structure.
2). Significance of holes in semiconductor
When compared to electrons, the significance of holes in semiconductors is that they can also be referred to as a carrier of electricity.
The mechanism by which holes carry electricity differs slightly from that by which electrons conduct electric current.
A hole exists in a semiconductor crystal when there is an incomplete bond. Because it is easier for the electron to form bonds with nearby atoms, it leaves its original position to occupy the newly created hole nearby.
When an electron moves to fill a hole left by its previous position in bond, it creates a new hole.
When the second hole is formed, an electron from any other neighbourhood bond may emerge to fill the second hole while also forming a new hole behind it.
As a result, it is possible to visualise holes moving in the opposite direction of electrons. As a result of these two types of electricity (or) charge carriers, semiconductors conduct electricity (electrons and holes).
If firmly think, it may visualise that as a hole moves in one direction, an electron moves in the opposite direction. When holes travel ahead, negative charge moves backward.
Carrier Concentration in Semiconductor
Positive charge moves forward while negative charge moves backward. Hence, a semiconductor crystal hole movement carries a positive charge.
An ideal semiconductor crystal creates as many holes as electrons per unit time.
When the temperature increases, the rate of making electron-hole pairs starts increasing, and when the temperature decreases, the number of electron-hole pairs decreases gradually because electrons and holes recombine in the crystal.
The hole’s mobility in the crystal is μh and that the electron’s mobility in the same crystal is μe.
Holes and electrons go in different directions. The electrons always move in the opposite direction of the electric field that is being applied.
The current density due to the movement of holes is given by
Jh=epvh=epμhE
The current density is expressed by the equation
Je=enve=enμeE
As the drifting of holes adds current in the same direction and the drifting of electrons adds current in the opposite direction, the currents are always in the same direction, which is in the direction that the holes are drifting. So, the total current caused by these two charge carriers will be the sum of the two currents, and the total current density will be,
J=Jh+Je = epμhE+enμeE = (pμh+nμe)eE = σE
Where,
σ- the conductivity of the semiconductor.
n – the concentration of free electrons and
p – the concentration of holes.
What is doping in semiconductors ?
Metal conductivity is increased by the addition of an equivalent amount of suitable impurity. This is referred to as doping.
It can be done with an impurity that is either more electron-rich or less electron-rich than the intrinsic semiconductor silicon (or) germanium.
Which semiconductor is the most conductive?
According to research, a material known as – cubic boron arsenide has two significant advantages over silicon. It has high electron and hole mobility as well as a good thermal conductivity. According to the studies, it is the best semiconductor material ever noticed.
In which semiconductor the conductivity is very high?
Extrinsic semiconductors have higher conductivity than intrinsic semiconductors. This is because the carriers in an intrinsic (pure) semiconductor are only thermally generated.
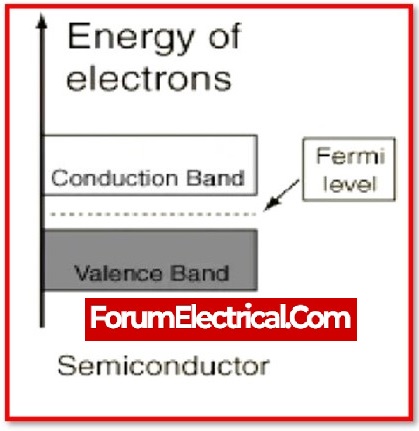
What is the energy gap (bandgap) of semiconductors?
A band gap, also known as an energy gap or band gap, is a range of energy in a material when there are no electron states present.
When temperatures rise, there is a general pattern for the energy gap of semiconductors to narrow down.