- What are Ferromagnetic Materials?
- Types of the Ferromagnetic Materials
- Properties of the Ferromagnetic Materials
- Advantages of the Ferromagnetic Materials
- Disadvantages of the Ferromagnetic Materials
- Applications of the Ferromagnetic Materials
- What is the function of curie temperature in ferromagnetic materials?
- Magnetic hysteresis in Ferromagnetic materials
- Causes of Magnetic hysteresis in Ferromagnetic materials
- Coercivity in Ferromagnetic materials
What are Ferromagnetic Materials?
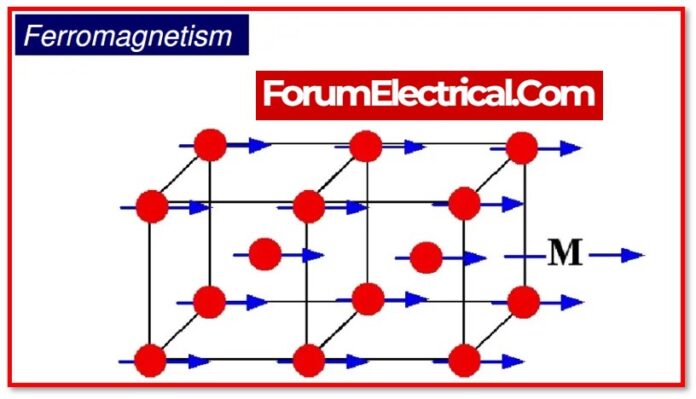
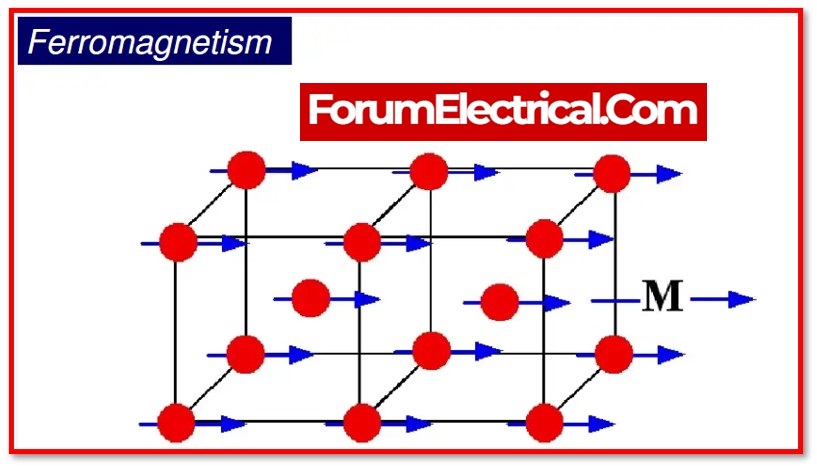
When a magnetic field is applied to a material, ferromagnetic substances display high magnetism in the same direction as the field. First, it is necessary to define a domain.
Due to a quantum mechanical process, it is a microscopic region in ferromagnetic materials with a particular overall spin orientation.
This impact is in fact an exchange interaction. In other words, when we examine unpaired electrons, they interact with one another between two atoms & align themselves in a limited area along the magnetic field’s direction.
This mechanism of the ferromagnetic substance is referred to as ferromagnetism. By the application of a magnetic field, some materials (cobalt, gadolinium, iron, etc.) become permanent magnets.
1). Cobalt
Georg Brandt found cobalt in 1739. He was born in Riddarhyttan on June 26, 1964, & died in Stockholm on April 29, 1768. It is a ferromagnetic substance found in the earth’s crust. On the periodic table, it is represented by the symbol CO, & its atomic number is 27.
2). Iron
Iron is the only chemical element found in the earth’s crust, and it is often symbolized by the symbol Fe. Iron has the periodic table atomic number 26 and the color silvery grey. Henry W Seeley created the first electric iron in 1882, which was used to iron garments. Henry W Seeley was born on May 20, 1861 in New York & died on May 20, 1943.
3). Nickel
The chemical element nickel, which is symbolized by the symbol Ni, is also found in the earth’s crust. Nickel has an atomic number of 28 in the periodic table & the color silvery white. Axel Fredrik Crostedt created this metal; he was born in Sweden on December 23, 1722, and died on May 20, 1943.
4). Neodymium Magnet
It is a sort of powerful and permanent magnet that is seldom found in the earth’s crust, and its hue is silvery white. It is also known as an NIB, Neo, or NdFeB magnet, and its formula is Nd2Fe14B. Carl Auer Von Welsbach created this metal; he was born in Austria on September 1, 1858 and died on August 4, 1929.
5). Chromium dioxide
Chromium dioxide with the chemical formula CrO2, is insoluble in water, and is also known as Chromium (iv) oxide. Carolyn and magtrieve are two additional names for Chromium dioxide. Louis Nicolas Vauquelin discovered the metal chromium. He was born in Austria on May 16, 1763 and died in France on November 14, 1829.
6). Gadolinium
Gadolinium is a chemical element that is represented by the symbol Gd. Gadolinium has the periodic table atomic number 64. Paul-Emile Lecoq de Boisbaudran (18th April 1838 – 28th May 1912) in France & Jean Charles Galissard de Marignac (24th April 1817 – 15th April 1894) in Switzerland developed the metal gadolinium.
7). Terbium
Terbium is a kind of chemical element that is denoted by the symbol Td. Carl Gustaf Mosander created it in 1843, and it is only sometimes discovered in the earth’s crust. Carl Gustaf Mosander developed this chemical element in 1843. He was born on September 10, 1797 in Kalmar and died on October 15, 1858 in Stockholm County.
8). Dysprosium
Dysprosium is a kind of ferromagnetic substance discovered in 1886 by Paul Emile Lecoq de Boisbaudran. He was born on April 18, 1838, and died in France on May 28, 1912. Gadolinium has the periodic table atomic number 66.
Types of the Ferromagnetic Materials
There are two kinds of ferromagnetic materials:
1). Un-magnetized ferromagnetic material and
2). Magnetized ferromagnetic material.
1). Un-magnetized ferromagnetic material
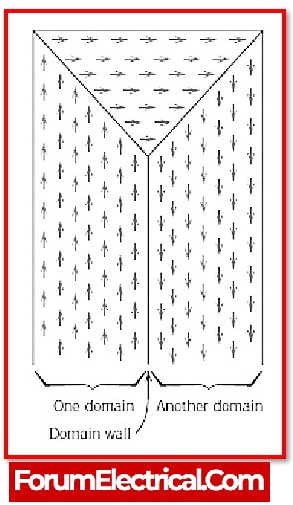
Within of each and every ferromagnetic material that has un-magnetised, the atoms arrange themselves into domains. The magnetic moment is aligned in a variety of different orientations throughout the various domains. As a result, the materials remain un-magnetized.
2). Magnetized ferromagnetic material
When an external magnetic field is supplied to the domains of un-magnetized ferromagnetic material, the domains spin and align in the direction of the magnetic field, since the domain character of the ferromagnetic material causes substantial magnetization even when a tiny magnetic field is applied.The magnetic field is much greater than the magnetic field that is present in such material. In ferromagnetism, the magnetic moments of domains align in the same direction as the magnetic field, which is why the magnetic moments of domains in ferromagnetism are parallel to the magnetic field.
Properties of the Ferromagnetic Materials
- The magnetic field significantly attracts ferromagnetic material.
- Even in the absence of a magnetic field, some substances exhibit persistent magnetism.
- When ferromagnetic materials are heated to high temperatures, they become paramagnetic.
Advantages of the Ferromagnetic Materials
- The level of resistance is high.
- The cost of hysteresis loss is modest.
- The electrical resistivity is great, while the coercivity is low.
- Permeability is quite high.
- It can withstand temperatures of up to 3000 C.
- Ferromagnetic materials provide high stability.
Disadvantages of the Ferromagnetic Materials
- Generates a very weak magnetic field.
Applications of the Ferromagnetic Materials
The ferromagnetic materials be used in the following applications:
- Transformers,
- Electromagnets
- Magnetic tape recording
- Hard drives
- Generators
- Telephones,
- Loudspeakers
- Electric motors
- Hard disk
- Magnetic Storage
There is a wide variety of materials that exhibit ferromagnetic properties; the table that follows provides examples of some of these materials.
| Serial Number | Ferromagnetic materials | Curie Temperature | Boiling Point (in Kelvin-K) | Melting Point (in Kelvin-K) | Atomic Number | Density (g/cm3) |
|---|---|---|---|---|---|---|
| 1 | Cobalt | 1388 | 3200 | 1768 | 27 | 8.91 |
| 2 | Nickel | 1043 | 3134 | 1811 | 26 | 7.87 |
| 3 | Iron | 627 | 3003 | 1728 | 28 | 8.901 |
| 4 | Neodymium Magnet | 593 | 3347 | 1297 | 60 | 7.61 |
| 5 | Chromium dioxide | 386 | 4273 | 648 | 24 | 4.89 |
| 6 | Gadolinium | 292 | 3273 | 1585 | 64 | 7.901 |
| 7 | Terbium | 219 | 3396 | 1629 | 65 | 8.23 |
| 8 | Dysprosium | 88 | 2840 | 1680 | 66 | 8.54 |
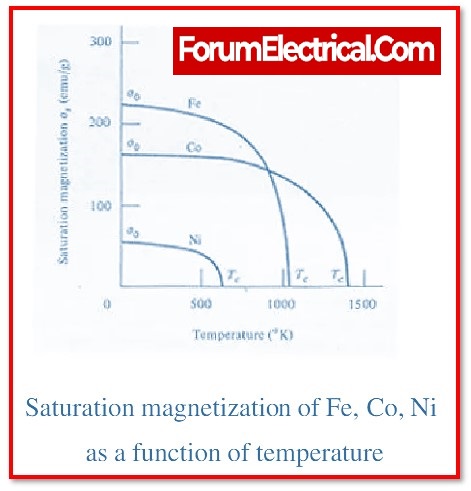
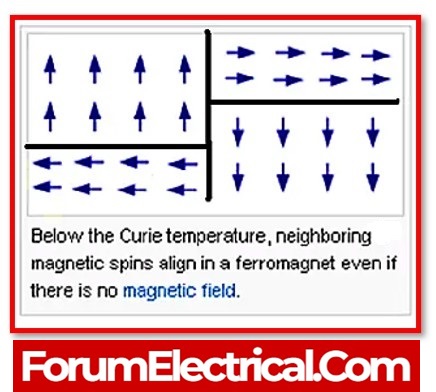
What is the function of curie temperature in ferromagnetic materials?
At a certain temperature, known as the Curie temperature for the material, the long-range order of a particular ferromagnetic substance suddenly disappears. Iron has a Curie temperature of around 1043 degrees Kelvin. The Curie temperature provides a rough estimate of the amount of energy that must be expended in order to destabilise the material’s long-range ordering. The thermal energy is around 0.135 eV when it is 1043 K, but it is approximately 0.04 eV when it is ambient temperature.
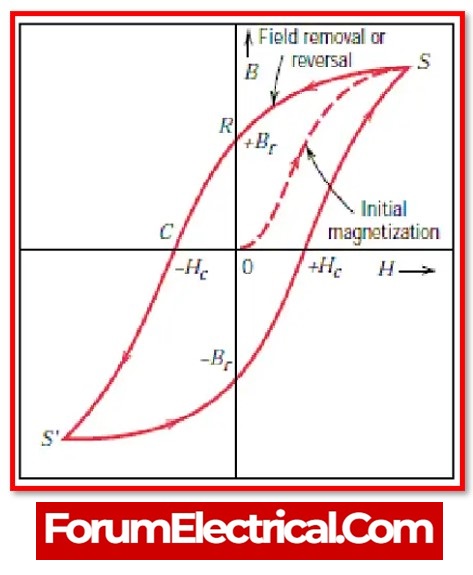
Magnetic hysteresis in Ferromagnetic materials
Magnetic hysteresis is most often seen in ferromagnetic materials.
When put in an external magnetic field for the purpose of magnetization, B exhibits a non-linear growth relationship with H. If H is reset to zero, the value continues to fall along the route ab.
This curve is referred to as the hysteresis curve since it follows B with H after it has already passed. (The term hysteresis refers to the delay that occurs between B & H.)
Causes of Magnetic hysteresis in Ferromagnetic materials
Residual magnetism is produced when an external magnetising field is removed (H = 0), since the magnetic moment of certain domains continues to be aligned in the direction in which the previous magnetising field was applied. This results in residual magnetism.
Residual magnetism = Br
Residual magnetism ☰ retentivity
Residual magnetism ☰ remanence
Once a magnetic field is removed from the ferromagnetic specimen, the amount of magnetic field that is still present in the sample is referred to as its retentivity.
Coercivity in Ferromagnetic materials
The amount of magnetising field that must be applied to a ferromagnetic material in order to eliminate any residual magnetism is referred to as its coercivity.