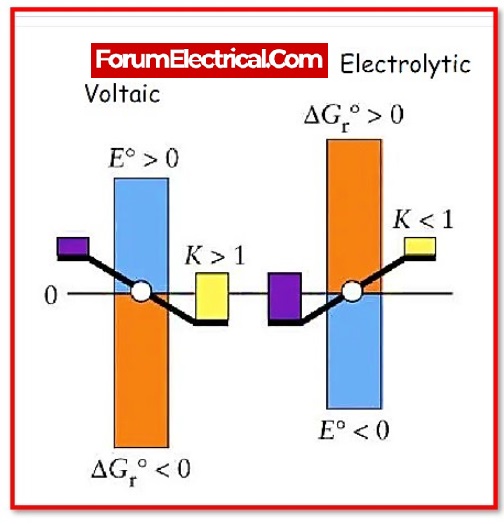
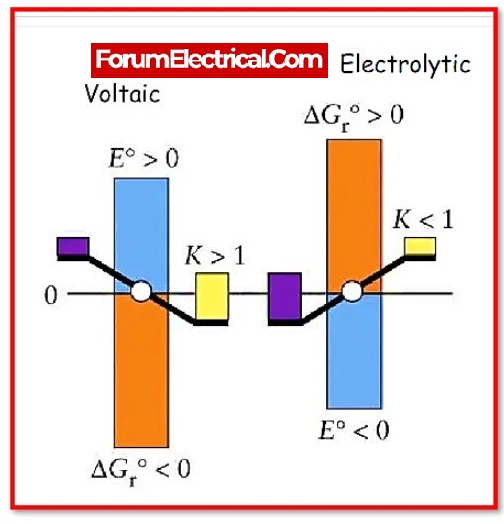
Faraday’s law of electrolysis is a principle in chemistry and electrical engineering that describes the relationship between the amount of electric charge that passes through an electrolytic cell and the amount of material that is produced or consumed at the electrodes. It is named after the English scientist Michael Faraday, who first described it in the early 19th century.
Faraday’s law of electrolysis Formula:
According to Faraday’s law, the amount of material that is produced or consumed at the electrodes of an electrolytic cell is directly proportional to the amount of electric charge that passes through the cell. This relationship is described by the following equation:
m = Q / zF
where:
m is the mass of material produced or consumed at the electrodes (in grams)
Q is the electric charge that passes through the cell (in coulombs)
z is the valence of the material (the number of electrons transferred per ion)
F is the Faraday constant, which is a physical constant that relates the amount of electric charge to the number of moles of material produced or consumed.
Faraday’s law of electrolysis is a fundamental principle in chemistry and is used to predict the behavior of electrolytic cells and to understand the relationships between electric charge, current, and chemical reactions. It is also an important concept in the field of electrochemistry, which studies the relationships between electricity and chemical reactions.